
Bowhead whales appear to have a DNA-repair mechanism superior to those of other species of mammal. This could explain why they live so long
A long life and staying healthy into old age — who wouldn’t want that? Nature seems to have bestowed bowhead whales with just this. As a species, they live longer than any other mammal — up to 200 years, and they apparently very rarely contract cancer. A research team led by the University of Rochester has now deciphered how they manage to reach such an advanced age.
Large animals with a long lifespan and a higher number of cells and cell divisions can be expected to accumulate a large number of DNA mutations during their lifetime. This should lead to a higher incidence of cancer and a shorter lifespan than in small animals. But the opposite is true. A phenomenon known as the Peto paradox. Thus, large animals such as bowhead whales must have particularly effective genetic mechanisms that prevent mutations from causing cancer or other age-related diseases.
One such mechanism is tumor suppression, but this is not sufficient to effectively and permanently prevent the development of cancer cells in long-lived animals. What makes the difference in bowhead whales are more precise and efficient repair mechanisms of something known as DNA double-strand breaks (DSBs), the research team found in experiments with fibroblasts, connective-tissue cells. The researchers identified two proteins, RPA2 and CIRBP, that contribute significantly to the precise repair of DSBs.
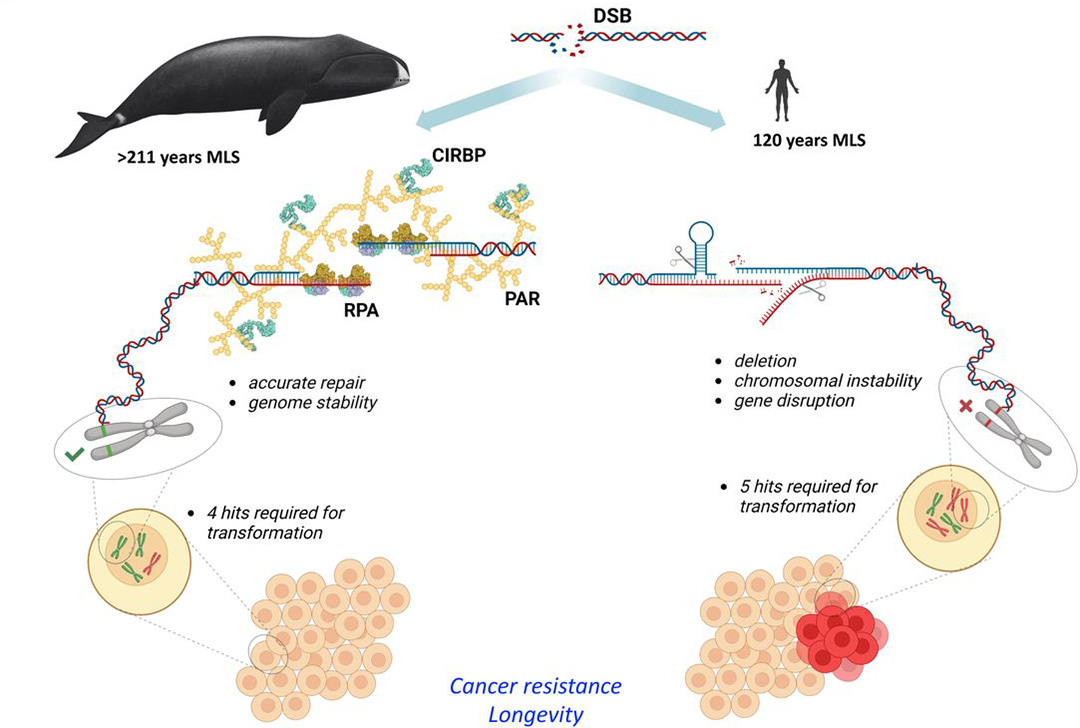
DNA double-strand breaks in bowhead whales are repaired with very high accuracy using the proteins RPA2 and CIRBP, unlike in humans (Illustration: Firsanov et al. 2023) 2023
According to the paper, which is currently awaiting peer review, the process of repairing rather than needlessly destroying cells could be critical to living a long and cancer-free life. Other species do not exhibit this type of DNA repair.
When breaks in DNA occur in cells “they are faced with a choice between several different fates, which co-operate to balance short-term risks to organismal viability with the long-term risks of genome instability,” the paper states. For example, DNA damage can be repaired and cell function resumed. However, a defectively repaired DSB carries the risk of causing cancer that is lethal to the organism, whereas an unrepaired DSB results only in the death of the cell. Therefore, mammals have evolved a variety of checkpoint mechanisms to block or eliminate cells with DNA damage.
If excessive or persistent damage occurs, cells may undergo one of two other processes — apoptosis, programmed cell death, or senescence, in which cell division is halted — that would be better for an organism’s survival and longevity.
Elephants, which live to a ripe old age of about 70, have found a trade-off between preventing cancer and age-related degeneration with tumor suppression and enhanced apoptosis. However, bowhead whales have developed the better strategy by shifting the balance from apoptosis and senescence to survival and precise repair.
According to the study, these efficient repair mechanisms are likely due, at least in part, to the high physiological stresses bowhead whales are exposed to in the extremely cold waters of the Arctic. Apparently, this causes a permanently high gene expression of the protein CIRBP.
In humans, the health-promoting effects of cold have long been known as a therapeutic agent. Perhaps we should all take the occasional cold bath to stay healthy for a long time.
Julia Hager, PolarJournal
Source
Denis Firsanov, Max Zacher, Xiao Tian et al. DNA repair and anti-cancer mechanisms in the longest-living mammal: the bowhead whale. bioRxiv 2023.05.07.539748; doi: https://doi.org/10.1101/2023.05.07.539748
More about this topic





